The Impact of PTEN Signaling on Neuronal Form and Function
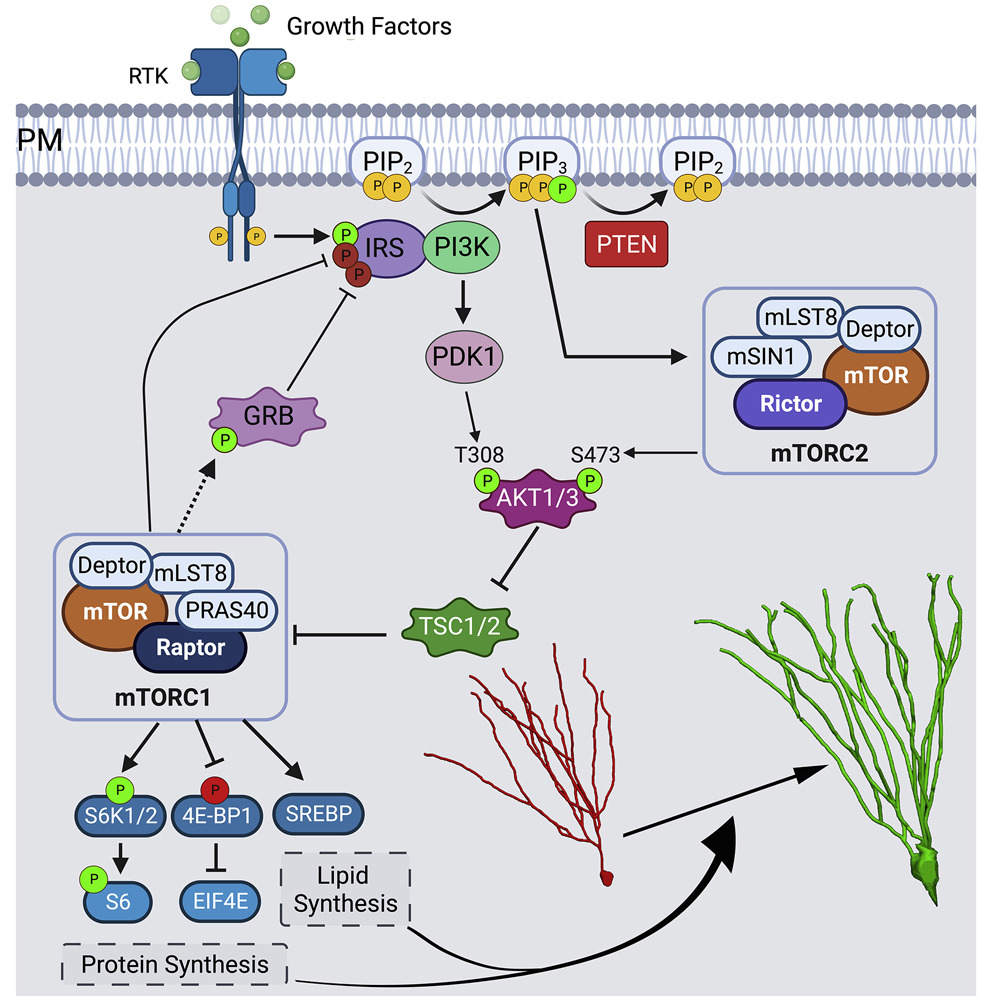
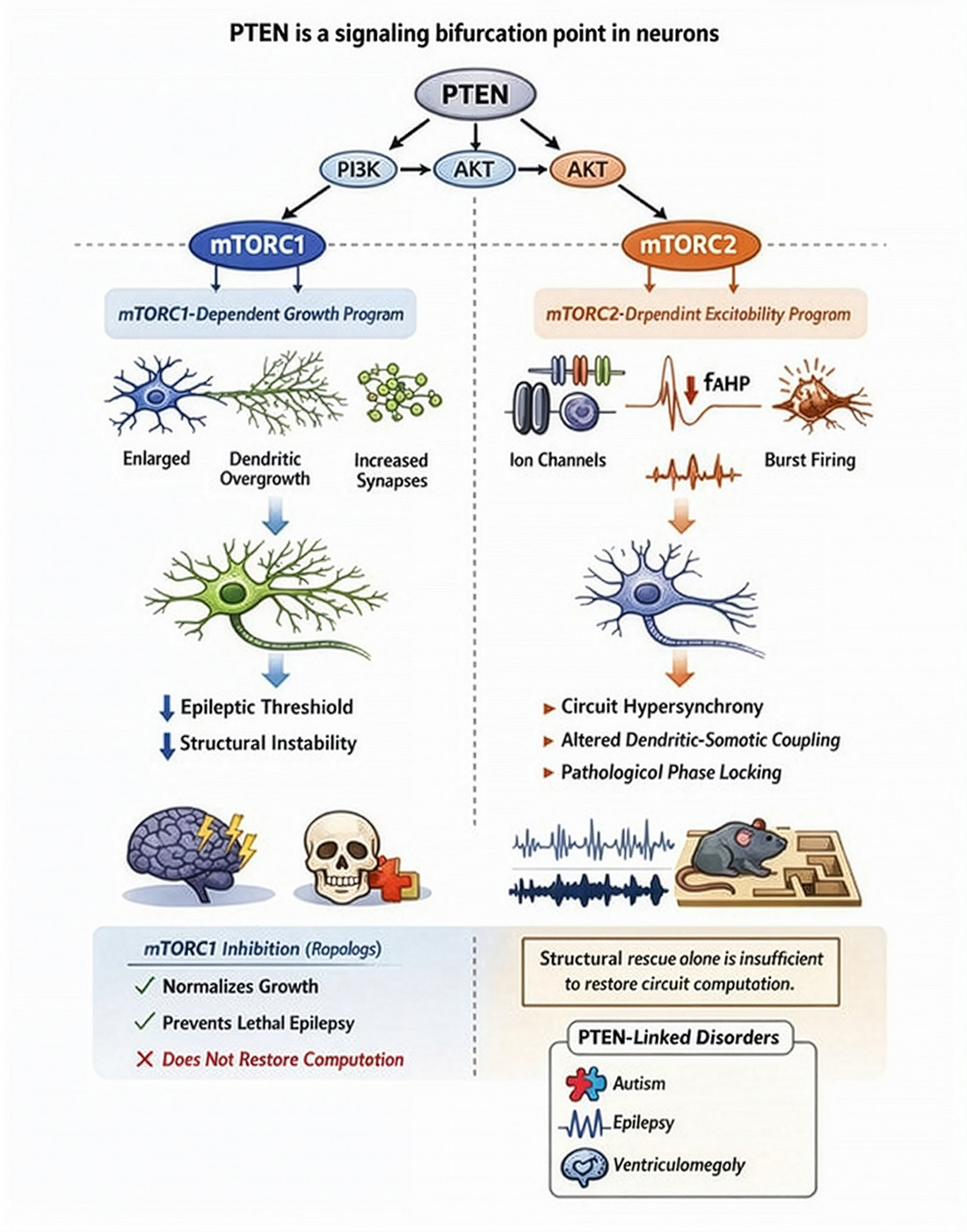
PTEN mutations are linked to autism spectrum disorder (ASD) and macrocephaly, and PTEN loss in mouse models drives excessive neuronal growth, increased synaptogenesis, and heightened excitability. We study how PTEN constrains PI3K signaling to preserve activity-dependent regulation of dendritic growth and synapse formation during development. Using intersectional mouse genetics and viral targeting of newborn neurons in vivo, coupled with imaging and whole-cell electrophysiology, we define signaling intermediates (mTORC1/mTORC2, AKT isoforms) and cytoskeletal mechanisms that govern dendritic elaboration, synapse formation, and neuronal function.